When a nonconformity occurs within a system, process, or product, the initial reaction individuals often have is to immediately correct the issue at hand. Although it is critical to ensure the nonconformity is addressed in an appropriate manner, it is also important to identify the potential causes
National Industrial Hemp Council Offers New Member Discount to Hemp Testing Laboratories for A2LA-NIHC Verify Joint Recognition Program
NIHC Verify Membership fee lowers to $325 per year for new hemp testing laboratories entering the program for the first two years Frederick, Md (October 29, 2024) – The American Association for Laboratory Accreditation (A2LA) and the National Industrial Hemp Council (NIHC) have announced a
Accreditation Case Study: PT Canada
Accreditation bodies, like A2LA, accredit PTPs to ISO/IEC 17043, the general requirements for the competence of proficiency testing providers, to provide third-party assurance and give customers confidence in the quality of the program. PT Canada (PTC), an A2LA accredited proficiency testing
Testing Candy for a Safe and Happy Halloween
Halloween is here and there’s no better way to celebrate the holiday than by enjoying a few sweet treats! Trick or treating is a fun, time-honored tradition dating back more than 100 years and is rooted in ancient customs surrounding community, spirituality, and prosperity. And, whether you go
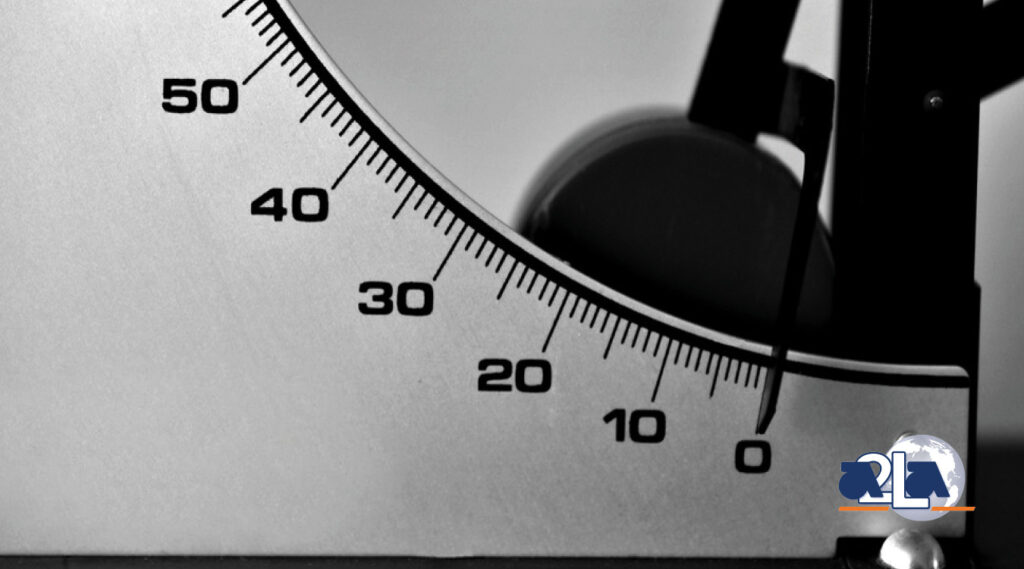
In-House Calibrations vs Internal Calibrations
Calibration laboratory personnel often use the terms “in-house calibration” and “internal calibration” interchangeably. Although they sound similar in meaning, these two terms have significantly different applications and using them interchangeably can often create confusion. It can also,
Celebrating World Accreditation Day with GE Grid Solutions
June 9th is World Accreditation Day, a day established by IAF and ILAC to recognize the importance of accreditation, a formal recognition earned by organizations proving they are qualified, competent, and comply with international standards. Not only does accreditation create confidence in the
QSM V6.0: What DoD/DOE Testing Labs Need to Know
Laboratories that participate in the A2LA Environmental Testing Accreditation Program are assessed to the requirements in ISO/IEC 17025:2017 and The NELAC Institute (TNI) standard requirements, through the National Environmental Laboratory Accreditation Program (NELAP). For laboratories that
Accreditation Case Study: Labcorp’s Center for Esoteric Testing and the Atlantic Division Regional Laboratory
Since its inception in 1995, Labcorp has grown to be one of the largest clinical lab providers in the world. Its flagship location in Burlington, NC is now Labcorp’s Center for Esoteric Testing (CET) and Atlantic Division Regional Laboratory (ADRL). This laboratory serves populations globally for