Imagine you’re the president of a testing organization with two separate labs. Each lab has its own quality management system (QMS), is accredited by different accrediting bodies (ABs) in two different languages, and there’s a nine-hour time difference between them. Operating these labs separately,
The Value of Accreditation
Accreditation is an attestation of a conformity assessment body’s (CAB) competence to perform conformity assessment activities based on a defined scope by an accreditation body, such as A2LA. It demonstrates competence, impartiality, and capabilities of CABs, thereby delivering confidence in goods
Building a Quality Culture at the Laboratory
Quality culture is built on the foundations of trust, active participation, and effective communication, where the achievement of quality objectives is cultivated through the involvement of employees. Culture is a learned behavior that is developed through interactions with other individuals within
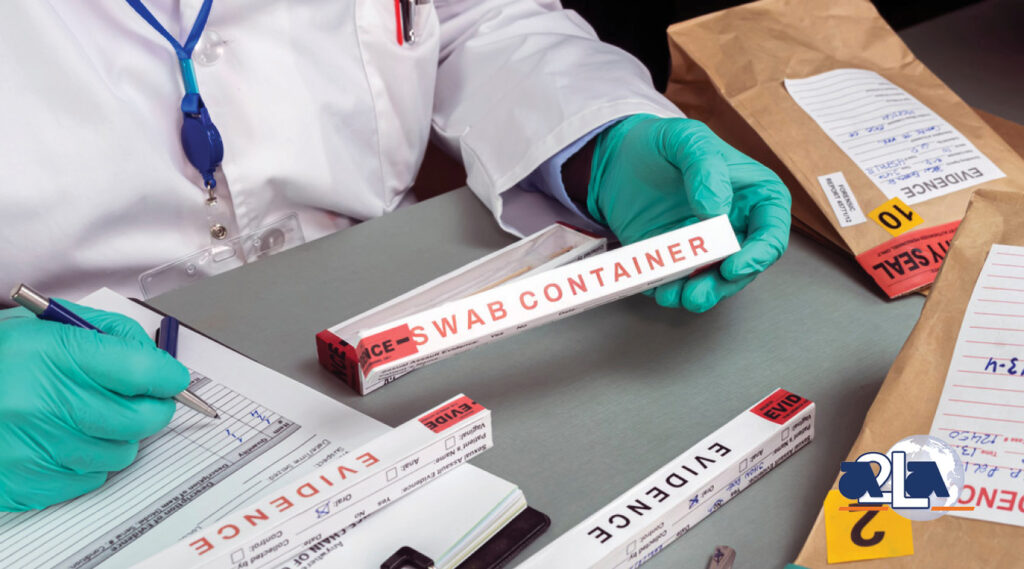
Sexual Assault Kits Initiative: Where Are We Now?
Since 2001, April has been the National Sexual Violence Resource Center’s Sexual Assault Awareness Month. The main goals of Sexual Assault Awareness Month are to raise awareness about sexual assaults and share information about how to help prevent it through education. It is also a time for
Laboratories & Calibration Providers: Let’s Talk
To read this article in Spanish, click here. A2LA accredits various laboratories and other organizations such as reference material producers, proficiency testing providers, inspection bodies, biobanks, and product certification bodies. A2LA is the largest accreditor of calibration
Root Cause Analysis Methods
Determining the root cause of a nonconformity is commonly referred to as root cause analysis (RCA). A root cause analysis is an important tool for continuous improvement and can be both the most challenging and the most essential part of the corrective action process. The purpose of a root
Common NGS Pitfalls of CLIA Certification and ISO Accreditation
A new DNA sequencing technology is transforming the world of genetic testing. This advanced technology is called Next-Generation Sequencing or NGS, and allows scientists, analysts, and geneticists to process millions of fragments of DNA simultaneously, as opposed to one sequence at a time. NGS
10 Steps Toward Achieving ISO/IEC 17025 Accreditation
Achieving ISO/IEC 17025 accreditation is one of the best ways an organization can stand out among their competition. If you’re not already familiar, ISO/IEC 17025 is an internationally-recognized published standard that details the general requirements for the competence of testing and calibration