 While nobody likes it when their organization receives a nonconformance, they are an inevitable part of operating an organization, especially one accredited to ISO/IEC 17025. However, nonconformances can show up in our day-to-day lives, too, like when mistakes are made and things happen outside of the standards of life. Remember that car crash you were in when you were 18? This is an example of a nonconformance against the rules of the road! Really, nonconformances are all around us, and ultimately, it’s important to embrace them so that we can learn more about how to prevent them from happening again in the future.
While nobody likes it when their organization receives a nonconformance, they are an inevitable part of operating an organization, especially one accredited to ISO/IEC 17025. However, nonconformances can show up in our day-to-day lives, too, like when mistakes are made and things happen outside of the standards of life. Remember that car crash you were in when you were 18? This is an example of a nonconformance against the rules of the road! Really, nonconformances are all around us, and ultimately, it’s important to embrace them so that we can learn more about how to prevent them from happening again in the future.
When reviewing a nonconformance, one of the most important aspects to think about is what caused that nonconformance to occur. That car accident we mentioned earlier; perhaps the road was slippery, and you lost control of the wheel or one of the cars involved didn’t brake at a stop sign, causing an accident to occur. In a laboratory, maybe a technician didn’t provide the measurement uncertainty on a calibration certificate simply because they weren’t aware of the requirements to include this. Just like nonconformances, their causes come in all shapes, sizes, and severities.
Throughout this paper, we will discuss the importance of accurately determining the causes of a nonconformance in any situation, whether it’s an accredited activity or not. We will also discuss multiple tools that can be utilized to determine the causes and how to effectively implement those tools. With a comprehensive understanding of the causes of nonconformances, you can take more effective actions and avoid the same nonconformance in the future!
What are nonconformances and why are they important?
As defined by Merriam-Webster dictionary, a nonconformance is simply the failure to conform [1]. Conform to what, someone might ask? That is a great question! In life, at work, at school, or for ISO/IEC 17025:2017 accreditation, there is a standard for how things should be done and any deviation from that standard is, by definition, a nonconformance. The term ‘nonconformance’ seems severe in meaning, and is often intimidating, but when you take a step back and evaluate what a nonconformance is, one can realize that a nonconformance is purely just an error made.
Here is a real-life example that everyone can relate to: in the morning before work, you go to pour yourself a bowl of cereal and find that last night after cooking dinner, you forgot to put the milk back in the refrigerator. The milk is now warm and completely spoiled, so it can’t be used for your breakfast. This simple example is a nonconformance. The standard requirement is that milk needs to be refrigerated, and the error was that the milk was left out.
Now, let’s apply that same logic to a laboratory scenario. You are the quality manager at an ISO/IEC 17025:2017 accredited calibration laboratory tasked with reviewing calibration certificates for final approval before they are issued to the customers. Upon review of several certificates generated by the same technician, you realize that there is no measurement uncertainty listed. This would be a nonconformance because the standard requirement is to include measurement uncertainty on the certificates, and the technician did not.
While it might not feel great knowing that you left the milk out on the counter to spoil, or that a technician neglected to include measurement uncertainty on the calibration certificate, nonconformances can tell us a lot! Nonconformances highlight where standards have been deviated from, ensure that quality is maintained—both in a bowl of cereal and on the calibration certificate—and allow people and organizations to find opportunities to continuously improve their processes and become better than they were before.
The most important thing about nonconformances is how they are solved after they occur, and a proper solution can only be put in place once you have a comprehensive understanding of why the nonconformance occurred. That said, the very first step to resolving any nonconformance is to determine why it occurred.
ISO/IEC 17025 requires that the cause of the nonconformance be determined, but that is not the only reason it is important to identify causes [2]. Utilizing the example from earlier with the technician omitting measurement uncertainty on the certificate, let’s pretend a cause was not identified but a solution was put in the place. The solution implemented was that the template for the certificate was updated to include a spot for the measurement uncertainties. At face value, it seemed that this would minimize the risk of uncertainty being left off but during an effectiveness review three months after the new template was implemented, it was determined that the technician still was not including the uncertainty on the certificate.
During the second attempt to resolve the issue, the technician was spoken to in an effort to determine why this was still occurring, and it was discovered that they were not aware that uncertainty was required. Although uncertainty was placed on the template, it was never communicated to them that it was necessary, so they were removing that section from the template prior to sending the certificates for review. After a 10-minute conversation with the technician, it was ultimately determined that they were never trained to put uncertainty on the certificate, making the root cause of this issue a lack of training. With that knowledge, the second corrective action put in place was to train all technicians in listing uncertainty and update the procedure for issuing certificates to ensure it includes measurement uncertainty.
As shown by this example, a fix without a cause analysis did not fix the issue at all. Ultimately, the time and effort needed to resolve the issue was increased by the first failed attempt, the template was adjusted when it perhaps was unnecessary, and the quality manager was frustrated with the technician for continuing to make the same mistake! With proper cause analysis, the fundamental issues behind the nonconformance can be identified and resources can be utilized in the most efficient manner to avoid ineffective solutions.
Cause Analysis Tools
Just as there are many potential solutions to nonconformances, there are many ways a cause analysis can be done. Two commonly used tools for cause analysis are the ‘5 Whys’ and a ‘Fishbone Diagram.’
Perhaps one of the most straightforward methods of cause analysis is the 5 Whys. With this method, the nonconformance is reviewed and the question “why” is asked repeatedly (usually about 5 times) to ensure that the underlying causes of the issue are found. After answering each question, ask why again to further dig into the cause.
Utilizing the example of the omitted measurement uncertainty, we will walk through an example here:
Nonconformance: Measurement uncertainty was left off all calibration certificates submitted by John Doe.
Why: Why was there no measurement uncertainty on the certificate provided for review?
Answer: John did not include the uncertainty when creating their reports.
W: Why did John leave uncertainty off the certificate?
A: John stated that he was unaware of the requirement to include uncertainty.
W: Why was John unaware of the uncertainty requirement?
A: This requirement was never explained to John upon hiring and initial training.
W: Why was this not communicated to John during training?
A: The procedure created for issuing certificates does not include the requirement for including uncertainty, so when John was trained on the procedure this was not included in the training.
W: Why was this requirement not included in the procedure?
A: It was assumed that calibration technicians knew measurement uncertainty was required on certificates.
At the end of asking why, it is determined that the procedure was lacking due to an assumption on the knowledge of technicians and this caused John’s training to exclude a very important requirement. An update of the procedure, as well as training, was done to address the identified cause.
Another cause analysis tool is the fishbone diagram, also known as an Ishikawa diagram. This diagram is useful in analyzing nonconformances when there are potentially multiple causes.
To begin the diagram, draw a horizontal line as the backbone of the diagram. The nonconformance, also referred to as the problem statement, goes at the “head” of the fish on the righthand side. From there, several branches are drawn off of the horizonal line. These lines will connect the main categories of potential causes to the problem itself.
Within each category, further investigation then needs to be done to determine specific contributing causes for each category. Much like the 5 Whys previously discussed, you will need to continuously ask why this occurred, and how the specific categories could have contributed to the nonconformance occurring [3]. Using the same nonconformance in our previous examples, an example of a simple fishbone diagram is shown below:
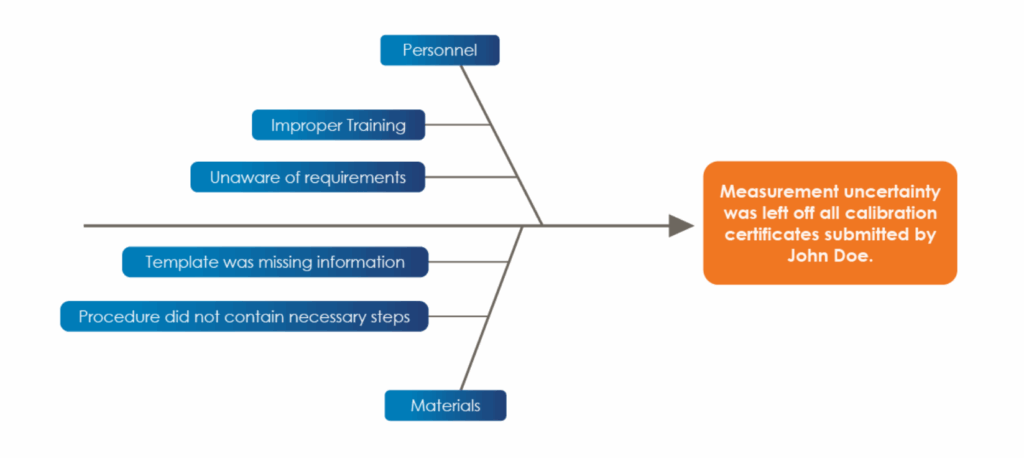
In the fishbone diagram example, the problem statement, or the nonconformance, is included at the head of the diagram, and the two categories that are being explored for causes are ‘personnel’ and ‘materials.’ Within each category, there are additional lines with potential causes. The example provided is a simplistic example of a fishbone diagram, however there can be many categories and factors to consider. It is important to note that a fishbone diagram often works best when a team of people contributes to the brainstorm. Remember, don’t work in isolation when trying to solve a problem!
After all the categories and potential causes are generated to satisfaction, the last step before implementation is to analyze the diagram you just created. Determine if there are any causes that require action, and keep in mind that more than one cause can warrant an action! If a fishbone diagram was utilized to determine the cause of the omitted uncertainty, it is likely that the template would have been updated, the technician would have been trained, and the procedure would have been updated all at once to rectify the issue.
Summary
As previously mentioned, nonconformances are inevitable but the most important thing is how they are handled once they occur. Cause analysis is arguably the most important part of solving a nonconformance, and special time and care must be given in the investigation of the cause. When you effectively determine the cause of nonconformances, efficient solutions can be implemented, reducing time, effort, and frustration when recurrence happens.
In this paper, we discussed two tools that can be used to determine causes but be aware those are not the only means of finding causes. There are several other tools and guides that exist to assist with cause analysis, and you’re encouraged to spend time researching those as well to determine the best fit for each situation. The bottom line is, it doesn’t matter how you arrive at the causes as long as you do.
References
1. “Nonconformance.” Merriam-Webster.com Dictionary, Merriam-Webster, https://www.merriam-webster.com/dictionary/nonconformance.
2. ISO/IEC 17025:2017: General requirements for the competence of testing and calibration laboratories
3. Tagaram SD, Chen C. Quality Tools and Techniques (Fishbone Diagram, Pareto Chart, Process Map) [Updated 2024 Sep 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan.
