 The transition to DoD/DOE QSM 6.0 has proven challenging for many laboratories, often taking longer than anticipated. In some cases, delays were due to higher numbers of nonconformances cited during assessments, while in others, laboratories simply were not prepared for the changes when their scheduled assessment occurred.
The transition to DoD/DOE QSM 6.0 has proven challenging for many laboratories, often taking longer than anticipated. In some cases, delays were due to higher numbers of nonconformances cited during assessments, while in others, laboratories simply were not prepared for the changes when their scheduled assessment occurred.
These challenges have been compounded by erroneous changes made to the B Tables for radiochemical testing, which have made it more difficult for laboratories to meet the required QC criteria.
In addition, the concurrent update from ISO/IEC 17025:2005 to ISO/IEC 17025:2017 has created added complexity for conformity assessment bodies (CABs). The shift from more prescriptive requirements to a risk-based approach has required significant adjustments in both interpretation and implementation, further slowing the adoption and full compliance with DoD/DOE QSM 6.0.
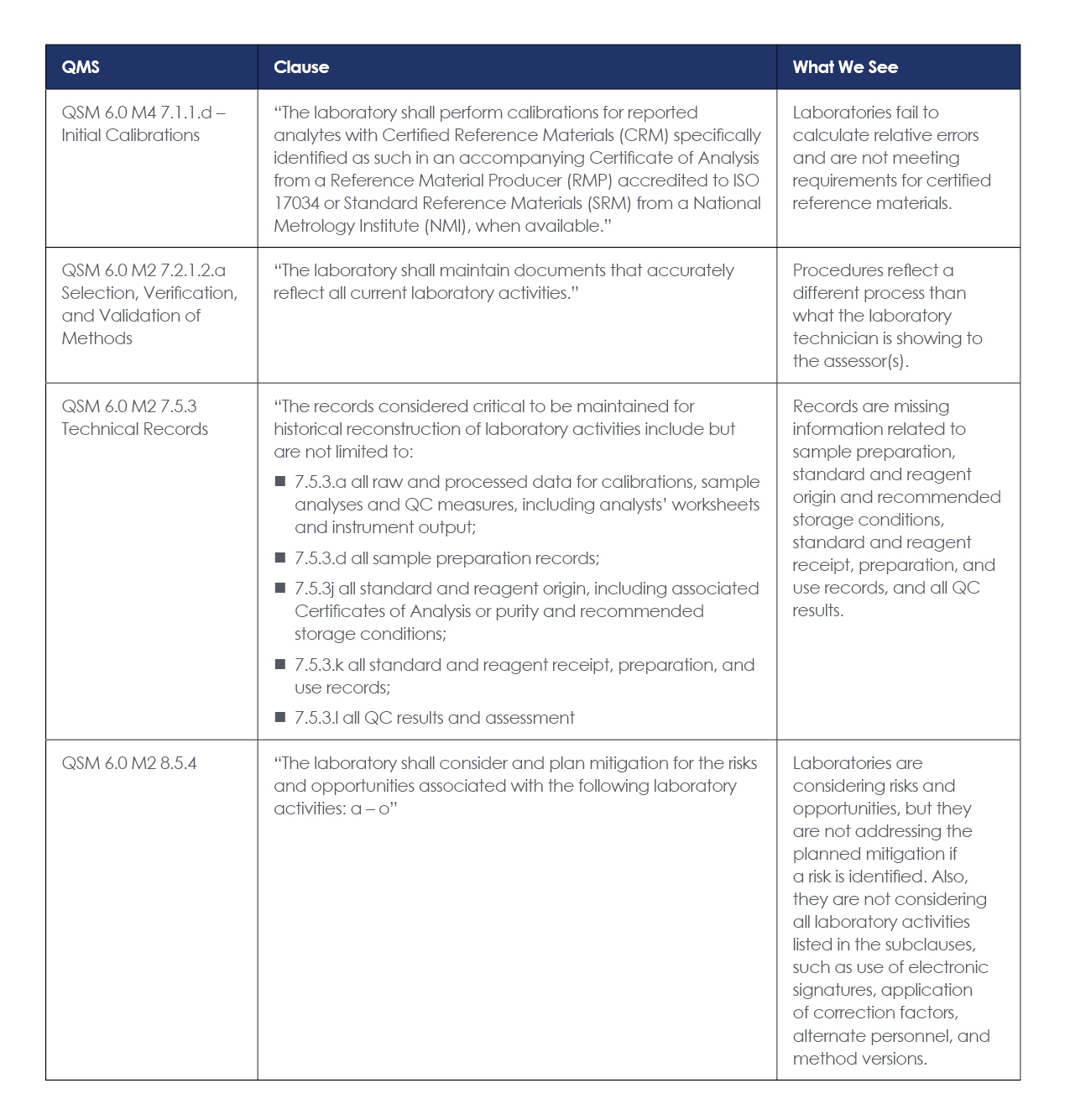
Laboratories were most often cited for the following four clauses, so let’s look at each and determine how to avoid them during your next audit or assessment.
Take the next step toward improving the quality of your organization’s test results. Request an accreditation estimate today.
