 Quality culture is built on the foundations of trust, active participation, and effective communication, where the achievement of quality objectives is cultivated through the involvement of employees. Culture is a learned behavior that is developed through interactions with other individuals within the organization and can become ingrained through rewards.
Quality culture is built on the foundations of trust, active participation, and effective communication, where the achievement of quality objectives is cultivated through the involvement of employees. Culture is a learned behavior that is developed through interactions with other individuals within the organization and can become ingrained through rewards.
A positive quality culture is important and necessary for the following reasons:
- Establishes policies and procedures
- Ensures consistency and reduces analytical drift
- Increases productivity
- Increases customer satisfaction
- Reduces risks and errors
- Ensures validity of results
- Promote continuous improvement
Mission, Vision, and Values
Many organizations realize that quality culture is imperative to its success. Therefore, it can be helpful to display your organization’s mission, vision, and core values on the website, in your office décor, and on employee handbooks to serve as a regular reminder to incorporate them into day-to-day operations.
For instance, A2LA upholds its core values which include quality, integrity, community, accountability, and leadership. They are displayed proudly on the A2LA website and throughout its headquarters in Frederick, Maryland. These values are exemplified in every employee and are evident in their activities. A2LA’s overall objective is to embody the highest level of integrity and expertise to create trust, safety, and quality throughout the world thereby creating a safer, healthier world.
Quality Management
Quality management is an area of expertise and quality leaders should understand all mechanisms within the quality management system including regulatory standards and accreditation requirements. It is important for quality leaders to understand that not all technical experts in the laboratory (i.e. laboratory technicians and examiners) are quality and accreditation experts. They are experts in their day-to-day operations, and they need to be educated on accreditation and the importance of quality assurance.
Team Building
An aspect of building a positive quality culture is team building, which involves a variety of activities aimed at team performance improvement. Team building activities can build and support:
- Team leadership
- Accountability
- Personal responsibility
- Clear objectives and goals
- Dedication and commitment
- Organizational support and resources
- Reward systems and recognition for team success
Integrating team building into the workplace culture offers numerous advantages, such as increased employee engagement in the laboratory, greater productivity and quality, clear communication between staff and management, and the cultivation of leadership skills among employees.
Laboratory management should note that if personnel involved in team building events do not observe an improvement within their organization, they may view their efforts as a waste of time. Consequently, this could result in a loss of trust, reduce motivation, and decrease morale and productivity.
Career Development and Empowerment
According to the U.S. Bureau of Labor Statistics, labor costs account for approximately 70% of total business expenses. With that being said, it makes perfect sense for a laboratory to invest in its staff’s professional growth with training and development opportunities, such as continuing education (i.e. courses offered by A2LA’s preferred training vendor, A2LA WorkPlace Training) and industry involvement.
Organizations should encourage their personnel to become active members in scientific organizations and attend industry events and conferences, including A2LA’s Annual Conference.
In addition, significant professional growth can be achieved through joining professional/scientific organizations. For example, at A2LA, subject matter experts are invited to become more involved in the accreditation process by serving as a member of a Technical Advisory Committee, on the Accreditation Council, and/or as an assessor.
Collectively, these opportunities can empower laboratory personnel to contribute not only to their organization, but as well as to the greater scientific community.
Recognition Programs
Recognizing and rewarding laboratory members who contribute to quality improvement can inspire others; therefore, acknowledging their achievements publicly and identifying those who go above and beyond can improve morale and increase productivity and overall quality. Supervisors should also document these achievements in their personnel’s performance evaluations, which in turn encourages and supports professional growth.
Corrective Actions and Needs for Improvement
Over the years, there has been an unrealistic development of a “Zero Errors” culture in the laboratory workplace. Organizations want laboratory staff to be successful, but the path to success may have moments of trial and error. Management and laboratory personnel should remind themselves that we are all human; it is not a matter of ‘if,’ but rather a matter of ‘when.’ Furthermore, identifying risk and opportunities for improvement are important in quality development.
Times of Change
Maintaining a positive quality culture is essential, particularly during periods of change which may include but are not limited to the following:
- Changes in management
- New technology
- Updated procedures/SOPs
- Switching from paper to electronic files
- Political and regulatory reform (such as local, state, or federal government)
The Kübler-Ross Change Curve Model demonstrates the emotional transitions in the stages of grief that laboratory staff might undergo during times of significant change.
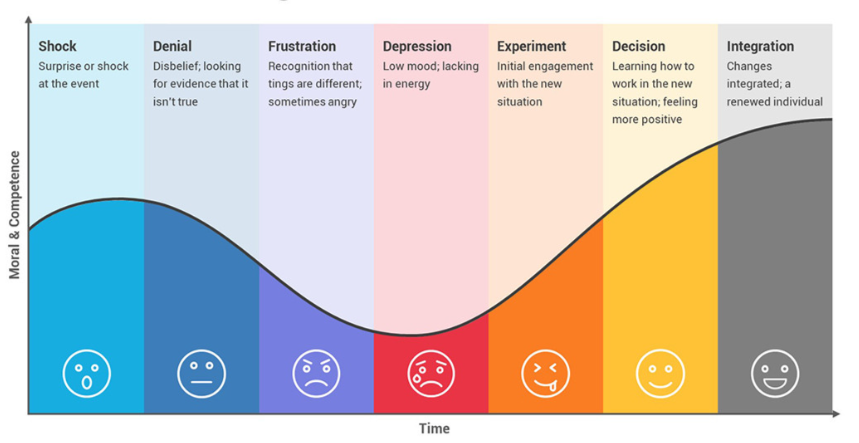
Changes within the laboratory can result in internal resistance and management should support their employees by providing guidance, maintaining clear and transparent communication, and offering support to help overcome these barriers. Productivity may decrease significantly during the depression stage, which indicates that the employees need an individualized plan to help navigate the remaining stages of emotions: experiment, decision, and integration.
Conclusion
Organizations like A2LA believe in going the extra mile for their employees and recognize that they are individuals with diverse goals, dreams, ambitions, and talents. A2LA staff work hard and enjoy each other’s company through participation in lunch-and-learn events, team-building events, and holiday celebrations. Their onboarding program is very welcoming to new employees, which assigns them a mentor and organizes lunches with fellow co-workers. A2LA has been officially recognized by Frederick County Maryland – Best Places to Work, Great Place to Work®, and Healthiest Maryland Business as a great place to work.

In 2024, Lawrence Livermore National Laboratory, a laboratory accredited by A2LA to ISO/IEC 17025 in the Chemical Field of Testing, was honored with a Glassdoor Employees Choice Award, which is based solely on the input of its employees who serve as scientists, engineers, business professionals, and technical staff.
“Being named as a ‘2024 Best Place to Work’ is a true testament of the lab’s commitment to sustaining an environment and culture that attracts, engages, and retains our stellar workforce,” Chief Human Resources Officer Larry Durham said.
By offering employees a clear mission statement, team building activities, regular recognition, and opportunities for personal and professional growth, organizations can improve their quality culture, putting them on the path to success.
Take the first step in building quality and confidence in your organization. Click here to request an estimate. For information about laboratory accreditation and building a quality culture in your organization, contact A2LA at info@A2LA.org.

