 Determining the root cause of a nonconformity is commonly referred to as root cause analysis (RCA). A root cause analysis is an important tool for continuous improvement and can be both the most challenging and the most essential part of the corrective action process.
Determining the root cause of a nonconformity is commonly referred to as root cause analysis (RCA). A root cause analysis is an important tool for continuous improvement and can be both the most challenging and the most essential part of the corrective action process.
The purpose of a root cause analysis is to mitigate future nonconformities. Understanding why an event occurred is the key to developing effective corrective actions. In some cases, the root cause is singular and easily discerned; in many cases it is not, and there may be multiple root causes. Because of this, there is no ‘one size fits all’ approach.
Below are some common root cause analysis tactics which can be employed to address two questions: “Why did this nonconformity occur?” and “How can this nonconformity be prevented moving forward?” It is important to keep in mind when answering these questions that the corrective action shall be intended to eliminate the root cause(s) of the nonconformity, so that it does not recur or occur elsewhere.
Fishbone Diagram
What is a Fishbone Diagram?
A fishbone diagram is a problem-solving tool utilized by an individual or organization that provides an opportunity to identify the root cause(s) of a problem, rather than the contributing factors. This tool is often used with the ‘5 Whys’ problem-solving tool.
Components of a Fishbone Diagram
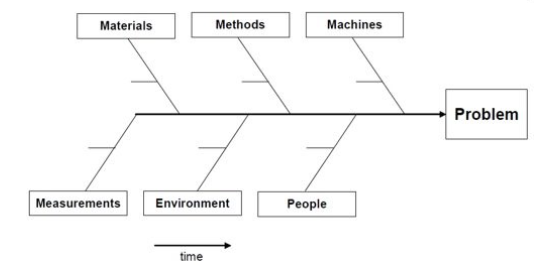 Problem Statement – identifies an issue within a process or procedure
Problem Statement – identifies an issue within a process or procedure
- Categories – the major or most obvious influences that affect your process or procedure; the main “ribs” of the diagram
- Contributing Factors – incorporates the use of the 5 Whys to help evaluate the potential root causes; the “ribs” of your categories
- Additional Factors – utilized on an as-needed basis; provide deeper insight into the causes of your contributing factors; the “ribs” of your contributing factors
- Root Cause – determines which contributing factor most influences the problem statement and provides explanation as to why an issue may be occurring
Drawing Conclusions from the Fishbone Diagram
When all brainstorming has been completed, the fishbone diagram is analyzed to determine any recurring causes between categories. These recurring causes are a good indication of the overall root cause and should be reviewed closely. If no recurring causes are present, team members should collaborate to determine which causes are the most influential to the problem. Once these causes are identified, collaborate further until an agreed root cause is identified. Once a root cause is identified, changes to a process or procedure can be put in place to prevent the issue from occurring again in the future.
Fault Tree Analysis (FTA)
What is a Fault Tree Analysis (FTA)?
FTA is a problem-solving technique used to ensure all factors contributing to a nonconformance are thoroughly reviewed. This can help the laboratory understand and address multiple potential causes of errors or failures that could affect the quality and reliability of test results, compliance with standards, or the overall functioning of the lab. This technique may be ideal when a problem has multiple contributing causes. It is also useful for complex systems, safety-critical systems, and evaluating interactions.
Components of a Fault Tree Analysis (FTA)
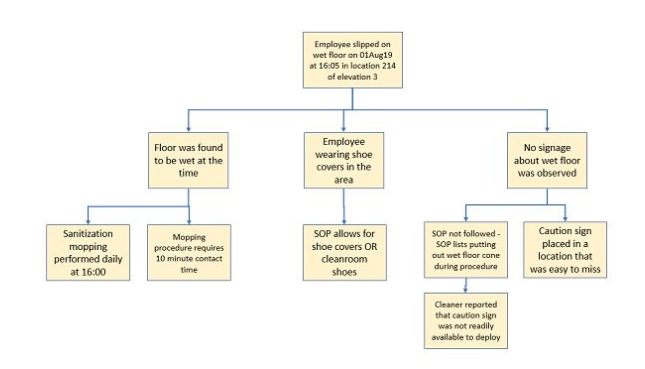 Layer 1 – Define the “top event” or primary failure to be analyzed. This could be failure of a PT test, noncompliance with a specific standard/clause, or failure to maintain equipment in a proper manner.
Layer 1 – Define the “top event” or primary failure to be analyzed. This could be failure of a PT test, noncompliance with a specific standard/clause, or failure to maintain equipment in a proper manner.
- Layer 2 – Construct the second layer of the Fault Tree or graphical representation of all possible contributing causes that could lead to the top event.
- Layer 3 and beyond – From the second layer, determine a third layer of cause analysis as shown below – this tree can be continued for multiple additional layers until the root of the problem is discovered, and should include at least three layers.
Drawing Conclusions from the Fault Tree
Once the fault tree is constructed, it is essential to assess and evaluate each cause in the tree. It is important to remember that there may be both primary and secondary causes stemming from one or more of the branches (causes). All primary and secondary causes should be evaluated for risks and prioritized to determine which/when corrective actions should be taken. To determine the main root cause in the FTA, identify the most critical path of nonsuccess and prioritize the corrective actions that solve the highest priority issue. After implementation, the corrective actions should be monitored and verified to ensure they are successful. All corrective actions and documents pertaining to the FTA should be retained for continuous improvement and record retention.
5 Whys
What is a 5 Whys analysis?
The 5 Whys is a tactic that can be employed by individuals and organizations as a starting place to determine the root cause for when a simple problem or a problem which is likely to have one root cause occurs. The steps are outlined below and begin with identifying the problem. ‘Embrace your inner toddler and keep asking why.’
This tactic provides limited information for the cause of multiplex problems with multiple independent contributing factors, however, can be critical when one variable leads to a nonconformance which is far-removed in location or time from the surveyor. While the number 5 is traditionally used, this could be simplified or extended. The 5 Why approach is mirrored and extended within the other tactics listed here.
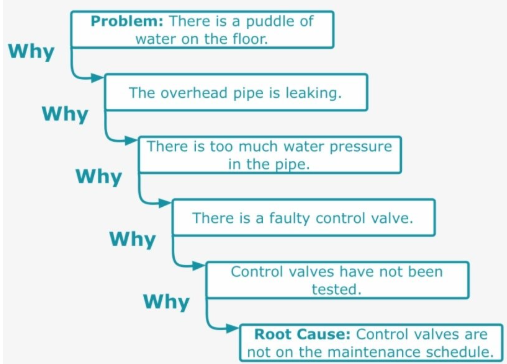 Components of a Five Whys
Components of a Five Whys
- Problem Statement
- First Why – Why is there a puddle of water on the floor?
- Second Why – Why is the overhead pike leaking?
- Third Why – Why is there too much water pressure in the pipe?
- Fourth Why – Why is there a faulty control valve?
- Fifth Why – Why have the control valves not been tested?
- Final Root Cause – Control valves are not on maintenance schedule
Drawing Conclusions from the 5 Whys
The conclusion is drawn after the final why is answered. To check that the root cause has been determined, ask yourself ‘if this final event had been addressed, would the entire nonconformity not have occurred?’ The answer should be yes. It is helpful at this stage to ask if any similar issues have occurred. For the example above, is there any other essential equipment which are not properly maintained on this schedule? This may help to identify and mitigate similar nonconformities.
Each of these techniques can be used to identify the source or sources of a problem prior to determining the action(s) to be taken. Remember, the corrective action implemented should address the root cause directly even when it appears to be potentially unrelated. For example, a problem with equipment might have a root cause which is related to insufficient training. In this example, the insufficient training should be addressed within the corrective action. This will prevent the problem from recurring.
An easy mistake to make is restating the nonconformity as the root cause statement. For example, for a nonconformity ‘Laboratory Management Review did not include an input of complaints,’ a restatement type root cause might be ‘Laboratory Management failed to include complaints as an input to the Management Review.’ This example statement does not provide insight as to why the problem occurred.
Some organizations may also misstep by oversimplifying or conversely overcomplicating the root cause analysis by either attributing one root cause to multiple issues (which stem from multiple causes) or conversely allocating significant time and resources to investigate and correct for a simple and preventable mistake. To simplify the process, each nonconformance should be evaluated independently, even in cases where one root cause results in multiple nonconformances. Taking this approach will make it easier to spot trends in the identified root causes for a group of nonconformances. For example, upon conclusion of an assessment during which 8 nonconformances were cited, it is determined that the root cause of 6 of the 8 nonconformances pertain to employee training. In this example, additional investigation into the employee training program would be prudent and should be evident in a response.
Take the first step in building quality and confidence in your organization. Click here to request an estimate. For more information about accreditation, contact A2LA at info@A2LA.org or call 301-644-3248.
