Accreditation proves an organization’s ability to perform work competently, which is why ISO/IEC 17025 places a heavy emphasis on personnel competency within section 6.2. This section of the standard contains several requirements—mainly covered in clauses 6.2.2 and 6.2.5—and it is one of the top ten areas in which A2LA cites nonconformities.
While clauses 6.2.2 and 6.2.5 are not inherently difficult to address, there are a few areas to consider when implementing these clauses into your management system. Requirements can and do fall through the cracks. In this document, we will cover the intent of each clause, and a few tips on how to address them.
ISO/IEC Clause 6.2.2
ISO/IEC 17025 clause 6.2.2 states:
“The laboratory shall document the competence requirements for each function influencing the results of laboratory activities, including requirements for education, qualification, training, technical knowledge, skills and experience.”
To better understand what this clause requires, it helps to think about a job posting. Your typical job posting will have the requirements you expect the prospective employee to bring to the table. On the same note, the ISO/IEC 17025 standard is asking you to put together a “job posting” for positions in your organization. These competency requirements should be documented and controlled within your quality management system. Typically, labs will put together a document that lists the job descriptions of each position in order to meet this requirement. A description of each competency requirement is listed below:
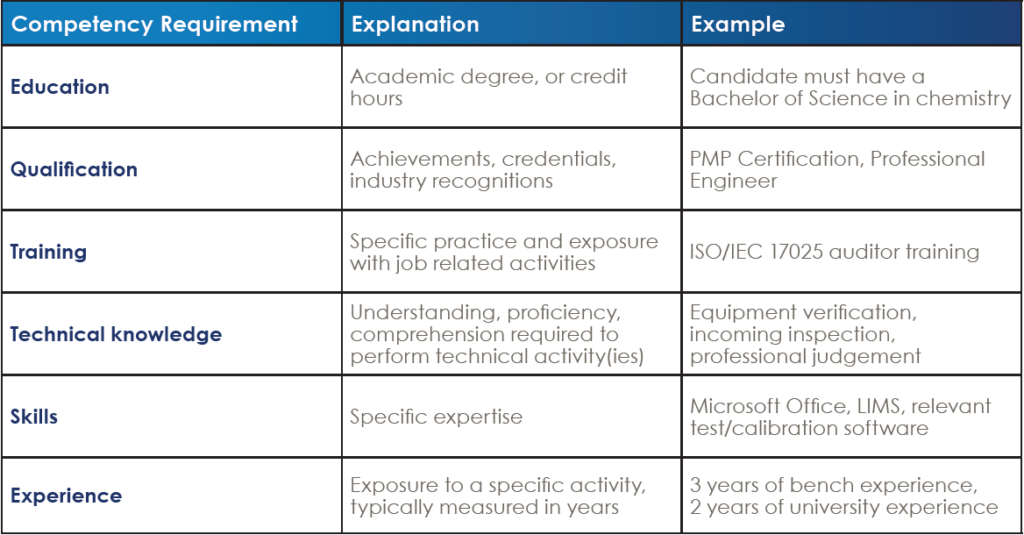
Note that not every position will require all of the above. An entry level position may require 0 years of experience. However, the competence requirement should still be addressed within your document.
- Technicians (e.g. performing the laboratory activities)
- Internal Auditor (e.g. confirming the compliance to requirements)
- Quality Manager (e.g. managing the quality system and ensuring consistent application)
- Sales (e.g. responsible for contract review)
- Shipping and receiving (e.g. responsible for handling test and calibration items)
In summary, as you document competence requirements, ensure you are considering all functions that affect laboratory activities with respect to the ISO/IEC 17025 standard.
ISO/IEC Clause 6.2.5
ISO/IEC 17025 clause 6.2.5 states:
“The laboratory shall have procedure(s) and retain records for: a) determining the competence requirements; b) selection of personnel; c) training of personnel; d) supervision of personnel; e) authorization of personnel; f) monitoring competence of personnel.”
This clause carries significant weight. Not only are there six separate requirements listed, but you must also have both procedure(s) and records for each area. As we break down each of these items, keep in mind the following:
- ISO/IEC 17025 only requires you (per clause ISO/IEC17025 5.5.c) to “document your procedures to the extent necessary to ensure consistent application.” Avoid overthinking your procedures!
- It is up to you whether you want a single procedure or multiple procedures to address these requirements. The requirements naturally flow into themselves, and as such, most organizations opt for a single procedure.
- Records can be checklists, spreadsheets, forms, workflows, etc. The standard gives you a baseline requirement and you make it work for your laboratory.
a) Determining the Competence Requirements
For this requirement, the standard is asking you to explain your process for putting together the competence requirements for each position related to your laboratory activities. To put it into perspective, if you were creating a new position for your organization, how would you go about determining the competence requirements per 6.2.2.? Would your HR department be responsible for that? Would you gather in a room and brainstorm? Whatever the process is, write it down. It can be as vague or detailed as you want. As long as it is consistently applied you are meeting the intent of the standard.
b) Selection of Personnel
Having your competence requirements determined makes the “selection of personnel” piece of the standard relatively straightforward. The competence requirements are the main aspect for determining whether an individual is hired into a position. That being said, there may be other areas outside of the competence requirements that you may want to consider, and that would be acceptable to include within your procedure.
c) Training of Personnel
Once personnel are selected, training is initiated. The standard does not specify what the training should entail. As such, it will be up to you to determine this.
The training may vary depending on the position. If you are hiring for a shipping and receiving clerk, the training could be very different than an individual responsible for calibrating equipment; however, both personnel will be expected to be competent in the relevant procedures and processes that affect the respective areas of the standard. The training program shall be robust enough to ensure that personnel are adequately trained to perform the work that could affect the laboratory activities.
d) Supervision of Personnel
During or after the training process, you may require supervision of your personnel prior to them performing the work on their own. If this is the case, you will need to document the process for supervising personnel. This may include aspects such as the length of oversight, what activities are supervised, and how this supervision is recorded. If you deem that the activity is low risk and therefore does not require supervision, be sure to still address supervision within your procedure.
e) Authorization of Personnel
Once personnel are deemed competent, they are authorized to perform the work they have been assigned without oversight. Within your procedure, you will specify what steps need to be taken to reach this point. For example, authorization may occur after 1 month of training and 2 weeks of supervision, or it may occur after 10 consecutive successful runs of a test.
Note: ISO/IEC 17025 6.2.6 has requirements for what personnel shall be authorized for.
f) Monitoring Competence of Personnel
As with several other areas of the standard, competency of personnel is not a “one and done” situation. Mistakes happen, procedures are forgotten, and as a result, consistency is lost over time. As such, ISO/IEC 17025 requires the competence of personnel to be monitored. It is up to the laboratory to determine how and when this occurs. For example, you may decide that this is done on an annual basis and through an annual training. Or, given the high risk surrounding your work, you may opt to monitor your personnel on a quarterly basis and utilize techniques such as peer evaluation, participation in proficiency testing, and supervision from upper-level management to monitor competence. Keep in mind that monitoring can vary between types of testing/calibration. Once the monitoring process is determined, document it in a procedure.
For items a-f, the records should reflect that you followed your process. If you fill out a training form to show that an internal auditor is authorized to perform internal audits, expect that record to be requested during an external audit.
The explanations given above may provide you with additional information to tackle the requirements of section 6.2.
Have more questions? Contact our Customer Care Team at info@A2LA.org or give them a call at 301-644-3248.
