When seeking accreditation to ISO/IEC 17025, a logical first step would be to read the standard and perform a gap analysis identifying which areas of the standard your organization may need to develop and implement. However, despite the best efforts of its authors, the standard isn’t exactly an easy read. While certain parts of the standard are written in a straightforward manner, there are also parts that are shrouded in ambiguity, which can require some analysis and interpretation to confirm what they mean and how they apply to your lab.
Before looking at the standard, it’s important to understand that the ambiguity is often by design. ISO/IEC 17025 is an international standard used for assessing competency for any and all types of testing, calibration, and sampling laboratories. Given all the potential varieties of laboratory organizations worldwide and their individual circumstances, some built-in flexibility is needed so that the requirements can properly accommodate all users while keeping to its core principles of assuring competency, impartiality, and consistency. Further to this point, ISO/IEC 17025:2017 was expressly written as a risk-based, process-approach standard. While it describes precepts and key elements, the depth of these items needs to be custom-tailored to the needs of each laboratory organization.
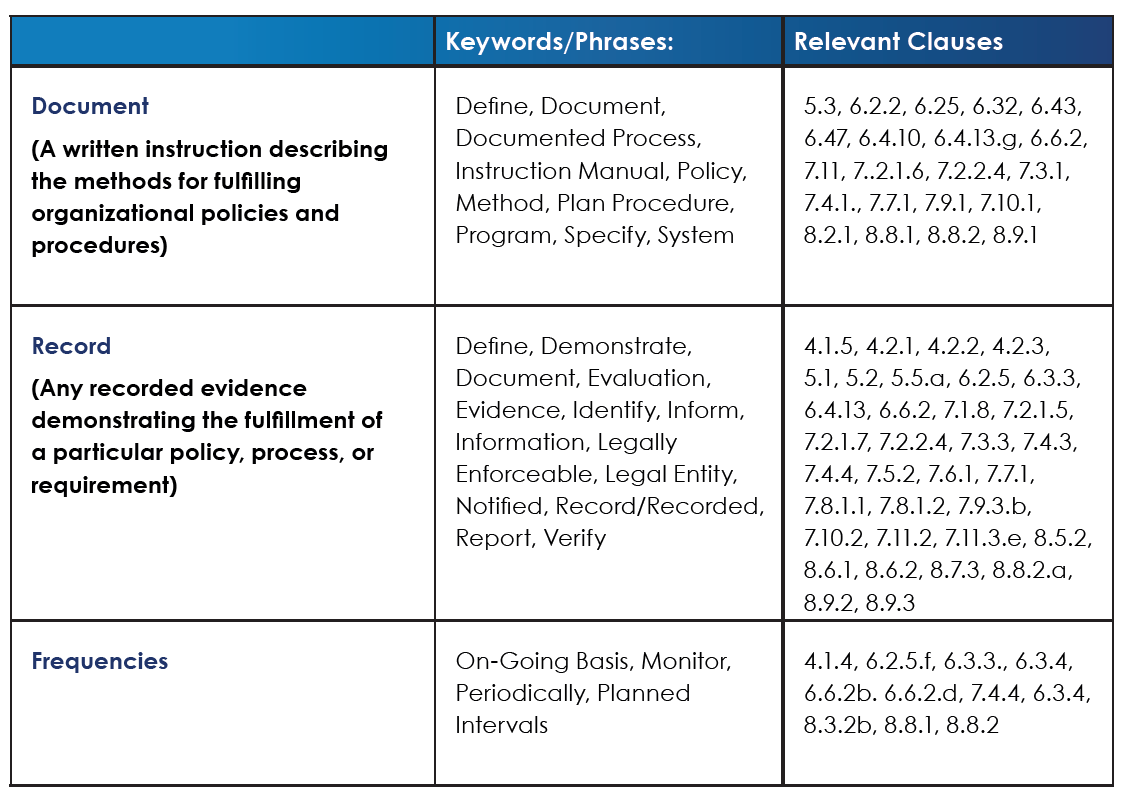
It can be helpful to approach ISO/IEC 17025 as a list of documentation and records requirements. Moving through the standard, you’ll see the requirements alternate back and forth on issues where evidence of a process is required. Fortunately, the standard is straightforward about its documentation (i.e. procedural) requirements and outright states which lab operations and activities need to be supported by a documented or written process. In other cases, the expectation is simply that the laboratory takes corresponding action on a given requirement. For all such cases, a laboratory must be able to produce a record demonstrating that appropriate action was taken. ISO/IEC 17025 begins with high-level, structural requirements in sections 4 and 5, before moving on to section 6 (resource requirements) and section 7 (process requirements for testing and calibration). The standard closes out with the management system requirements in section 8.
The below table lists some of the terminology you’ll encounter throughout sections 4 through 8 and identifies which clauses require written documents vs. those requiring records.