In This Issue…
- A2LA Launches ISO 20387 Biobank Accreditation Program
- A2LA Staff Participate in International Cannabis Standardization Event
- A2LA Achieves ISO/IEC 17011:2017 Recognition
- A2LA Documents: New Naming Convention
- ISO/IEC 17025:2017 Transition Reminder
- Cannabis Customer Spotlight
- New Employees and Milestones
- New & Updated Documents
► A2LA Launches ISO 20387 Biobank Accreditation Program
By: Kelsey Roberts, A2LA Marketing Manager
A2LA is proud to announce the launch of its new Biobank Accreditation Program. This third-party accreditation offers an independent review of an organization’s compliance to ISO 20387 – Technical and Quality Requirements for the operation of various types of biobanks/biorepositories for the desired scope of accreditation.
This new program will incorporate a review of the activities performed by biobanks to ensure proper collection/acquisition of material, authentication of the material, and the appropriate preservation and storage of the material.
If an organization is interested in becoming accredited to ISO 20387 with A2LA, the first step is to purchase a copy of the ISO 20387 standard, which was developed by leading experts in the biobanking industry. A2LA has identified qualified experts with direct experience in the type of biobank being operated to perform an on-site assessment of applicants. For more information or to apply for accreditation, please visit www.A2LA.org/biobanking.
► A2LA Staff Participate in International Cannabis Standardization Event
By: Roger Brauninger, A2LA Biosafety Program Manager
A2LA staff recently attended a trade show and participated in a workshop sponsored by ASTM technical Committee D37 on Cannabis. The workshop “Advancing the Field of Cannabis/hemp through Standardization” was held in Rome, Italy, immediately following the CanapaMundi International Cannabis Fair. This attracted participations from the United States, Canada, Columbia, the Netherlands, France, Germany, the United Kingdom, Cech Republic, Australia and New Zealand, among others.
The ASTM D37 committee has been working to craft market relevant standards for the global cannabis industry with the goal to develop standards for cannabis, its products, and processes that meet the needs of the legal cannabis industry by addressing quality and safety through the development of classifications, specifications, test methods, practices, and guides for cultivation, manufacturing, quality assurance, laboratory considerations, packaging, and security, etc. The objective of D37’s Workshop on Advancing the Field of Cannabis/hemp through Standardization was to energize the international community and solicit, not only participation from stakeholders, but also the creation of new standards and the appointment of regional liaisons responsible for updating the D37 committee on developments in their area.
► A2LA Achieves ISO/IEC 17011:2017 Recognition
By: Trace McInturff, A2LA Vice President, Accreditation Services
A2LA has been approved by both the Asia Pacific Accreditation Cooperation (APAC) (on March 6, 2019) and the InterAmerican Accreditation Cooperation (IAAC) (on February 20, 2019) for recognition to the newly revised ISO/IEC 17011:2017 – Conformity assessment – Requirements for accreditation bodies accrediting conformity assessment bodies which was released in November 2017. This marks A2LA as the first US AB to achieve international recognition to the new ISO/IEC 17011:2017 standard.
Through the APAC and IAAC resolutions, A2LA maintains its signatory status to the MRAs for accreditation of testing and calibration laboratories (ISO/IEC 17025), medical testing laboratories (ISO 15189), inspection bodies (ISO/IEC 17020), reference material producers (ISO Guide 34 / ISO 17034), and proficiency testing providers (ISO/IEC 17043).
This is a four-year recognition so the next full re-evaluation of A2LA will be scheduled by May 2022.
On behalf of the Association, I would like to thank our staff, assessors and CABs that were involved in this evaluation. We couldn’t have done this without you.
► A2LA Documents: New Naming Convention
By: Pam Wright, A2LA Quality Manager
A2LA is pleased to announce that we have upgraded our document management system with the purchase of Compliance Management Software from Qualtrax. This upgrade has allowed us the opportunity to streamline our management system processes and redesign our outdated and unmanageable document naming convention, making it easier to identify the documents you need. In order to provide a bridge between the old naming convention and the new one we will temporarily include a reference to the old document number in the title of the document. This will allow the document to be searched by either the new or old designation and provide for an easy transition.
The new naming convention consists of four parts:
- A prefix code (to identify document category);
- A unique document number (as assigned by the Qualtrax software);
- A document title that begins with the field of accreditation or general type (e.g. Cannabis, Forensic, Proficiency Testing, Policy, Accreditation);
- A temporary reference to the old document number.
The prefixes are defined as:
Abbr. ACS File
AP Application
AR Accreditation Requirements
SR Supplemental Requirements
PD Accreditation Policies
CK Checklist
ID Instruction/Information
FM Form
Some examples include:
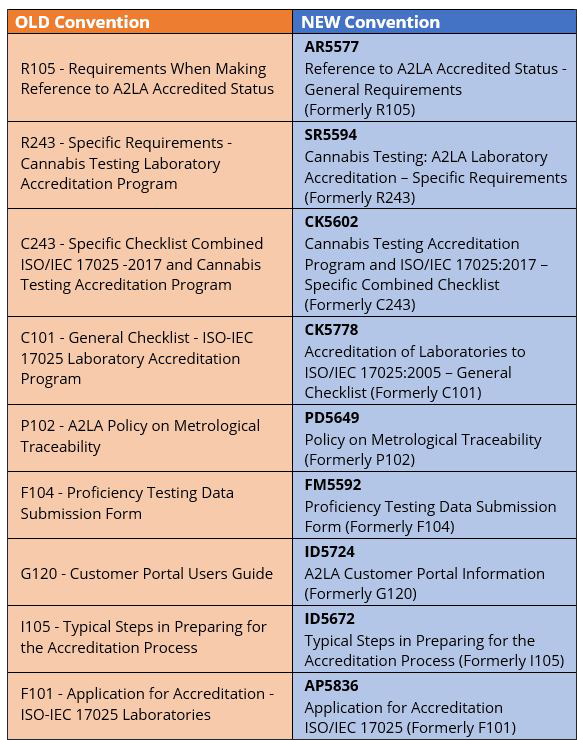
We would like to point out that all the previously designated Guidance documents are now placed under the Instruction/Information designation.
The newly named documents will be upgraded in the Customer Portal, Assessor Portal and on the A2LA Website on or around June 1, 2019.
► ISO/IEC 17025:2017 Transition Reminder
By: Trace McInturff, A2LA Vice President, Accreditation Services
As indicated in recent A2LA Today newsletters and our November 30, 2017 Transition Memorandum, we began assessing to the new ISO/IEC 17025:2017 standard on April 1, 2018. New applications received prior to September 30, 2018 have the option of being assessed to either the :2005 or the :2017 version of the standard. However, please note that any new laboratory assessed to the :2005 version of the standard will be required to undergo an ISO/IEC 17025:2017 delta assessment during their 1-year surveillance assessment to bring them into alignment with the new version of the standard. At this point, all renewal assessments will be assessed to the new ISO/IEC 17025:2017 standard. This is to ensure that A2LA meets the ILAC imposed three-year implementation deadline of November 30, 2020.
As of March 1, 2019, A2LA has converted 499 of 3241 (15%) ISO/IEC 17025 accreditations to the new :2017 version of the standard and has an additional 865 accreditations in process of being converted.
If you have any questions or need any additional information, please contact your Accreditation Services Officer.
► Cannabis Customer Spotlight
By: Anna Williams, Senior Accreditation Officer at A2LA
Cannabis Customer Corner: As the legalization of cannabis continues to be adopted by additional states across the United States, it is important to ensure that the public can be confident in the resulting products that they may now legally use. A2LA is committed to ensuring quality and compliance in this industry, as evidenced by our participation in industry groups, support of state regulations, online and in-person training, strategic partnerships, and publishing free educational content. To demonstrate why accreditation to ISO/IEC 17025 is important in the cannabis industry, as well as why A2LA’s cannabis testing laboratories have chosen A2LA as their go-to provider, we sought feedback from Alena Rodriguez, Managing Director, and Garrett Reese, Quality Manager, at Rm3 Labs Colorado LLC, an A2LA accredited cannabis testing laboratory.

1. Can you give me a summary of your company?
– Rm3 Labs, founded in 2009, is a Colorado state-certified and ISO/IEC 17025 accredited analytical cannabis testing laboratory. We provide compliance testing such as potency, homogeneity, residual solvents, pesticide, and microbial analysis. We also offer voluntary tests such as terpene and plant sex typing. We have assembled a team of top scientists and support staff to continually push the boundaries of cannabis science while never losing sight of our clients’ need for fast, accurate, and reliable results. We use our decade of experience and knowledge to help the cannabis industry create compliant, safe, and effective products.
2. How did your company get involved in the cannabis testing space?
– Rm3 Labs was created by Ian and Kelly Barringer, who early on saw a need for cannabis testing to ensure the safety, purity, and accuracy of Colorado’s cannabis.
3. Why did your company initially choose A2LA over another Accreditation Body?
– We chose to work with A2LA because of its superb reputation as an international accrediting body and because the organization has a cannabis-focused program to specifically fit the needs of cannabis testing labs. A2LA is the only Accreditation Body to offer optional cannabis-specific program requirements, giving labs a chance to add extra credentials to their scope and stand out from their competitors.
4. What are some reasons why you have remained an A2LA customer?
– We have remained an A2LA customer because of all the support we have received during the initial and surveillance assessments and the informational training offered by the organization.
5. What is one piece of advice you would give to a potential or current cannabis testing laboratory as they look into accreditation?
– I would advise cannabis testing labs to take one of A2LA’s training courses to better prepare them for the accreditation process.
► New Employees and Milestones
New Employee Announcements:
At the start of the new year, A2LA welcomed several new staff members to our growing team!

From left to right, front row: Soraya and Samantha. Back row: Heather, Nathan and Sarah.
• Soraya McClung joined the A2LA Training team as our new Technical Training Specialist. Soraya brings with her years of experience working in accreditation and training within the forensics industry. In some of her most recent roles, Soraya has been responsible for providing training to and consulting with organizations (nationally and internationally) who have pursued accreditation to ISO/IEC 17025 & 17020 through A2LA. Soraya holds a B.S. in Biochemistry and a Masters in both Biochemistry and Forensic Science.
• Ms. Samantha Hays joined A2LA as an Accreditation Officer on the IB & Materials team. Before joining A2LA, Sam had been working as a Quality Control Lead Analyst and providing support to a start-up lab through Proctor and Gamble (P&G) in West Virginia. She also has several years of experience working in a variety of customer service roles. Sam received her B.A. in Biology and Chemistry from Shepherd University, WV.
• Mr. Nathan Dula joined A2LA this year as a Business Development Associate. Nathan received his B.S. in Business Administration from the University of Louisville. Before joining A2LA, Nathan gained experience working in contract administration as well as several years spent as a technical recruiter.
• Ms. Heather McLemore joined A2LA as an Accreditation Officer on the Life Sciences team. Heather earned her B.S. degree in Food Science from Iowa State University. She brought with her several years of experience working in the lab at Tyson’s Fresh Meat as well as time spent at CTI Foods.
• Sarah Dorris came on board with A2LA on the Product Certification and Electrical team as a new Accreditation Officer. Sarah received her B.S. in Biology and more recently her Masters in Health Administration. Prior to joining A2LA, Sarah spent several years of her career working in the healthcare industry. In the past, she has also held a variety of customer service positions and spent time teaching in both high school and college level courses.
We are happy to have Soraya, Sam, Nathan, Heather and Sarah on our growing team and look forward to seeing where their A2LA career takes them!
If you or someone you know is interested in employment with A2LA, please refer to our career page to learn more about any available positions or sign up for our job alert notifications at www.A2LA.org/careers.
Employee Milestones:
January
• Karen Rudd, Office Coordinator – 20 Years
• Ashly Carter, Program Manager, Calibration – 10 Years
• Zach Keyser, Accreditation Officer, Product Certification and
Electrical – 1 Year
• Carrie Whitaker, Quality Manager – 1 Year
• Dyana Cernicky, Accreditation Officer, Product Certification and
Electrical –1 Year
• Cory Arant, Accreditation Officer, Life Sciences – 1 Year
February
• Roger Brauninger, Program Manager, Biosafety – 20 Years
• Jordan Acton, Program Manager, Product Certification – 5 Years
• Carlyn Brecht, Accreditation Officer, Life Sciences – 1 Year
March
• Chris Gunning, Accreditation Manager, Life Sciences –
10 years
• Amber Ryan, Accreditation Officer, Calibration – 1 year
► New & Updated Documents
By: Pam Wright, A2LA Quality Manager
The following documents have been updated within the controlled A2LA management system. These documents are available on the A2LA website (www.A2LA.org) through the “Search” tool or may only be available on your customized portal (as appropriate) unless otherwise indicated.
If you have any questions about these updates, please contact A2LA at 301.644.3248 or your Accreditation Officer.
Checklists:
A311a – RSS and BETS Scope Confirmation Form is a revised document dated 2/13/2019. Editorial for the addition of Part 2 and Updated RSS 130 and RSS 196 Issue references.
C204 – Specific Checklist – Combined ISO/IEC 17025 and Food, Dietary Supplements and Pharmaceutical Testing Laboratory Accreditation Program Requirements is a revised document dated 1/8/19. Non-Editorial for the addition of AOAC 2017 clauses in italics (CAB Portal Only).
C232 – Specific Checklist – Combined DoD_DOE QSM Version 5.2 and NELAC TNI Standard Module 3-Quality Systems for Asbestos Testing is a revised document dated 1/22/19. Non-Editorial for added new requirements and updated requirements as published in the DoD/DOE QSM Version 5.2 (CAB Portal Only).
C233 – Specific Checklist – Combined DoD_DOE QSM Version 5.2 and NELAC TNI Standard Module 4-Quality Systems for Chemical Testing is a revised document dated 1/22/19. Non-Editorial for added new requirements and updated requirements as published in the DoD/DOE QSM Version 5.2 (CAB Portal Only).
C234 – Specific Checklist – DoD QSM Version 5.2 Module 1-Proficiency Testing is a revised document dated 1/22/19. Non-Editorial for added new requirements and updated requirements as published in the DoD/DOE QSM Version 5.2 (CAB Portal Only).
C235 – Specific Checklist -Combined DoD_DOE QSM Version 5.2 and NELAC TNI Standard Module 6-Quality Systems for Radiochemical Testing is a revised document dated 1/22/19. Non-Editorial for added new requirements and updated requirements as published in the DoD/DOE QSM Version 5.2 (CAB Portal Only).
C245 – Specific Checklist – Combined ISO-IEC 17025, NELAC TNI Standard Module 2 and DoD_DOE QSM Version 5.2 Quality System Requirements is a revised document dated 1/22/19. Non-Editorial for added new requirements and updated requirements as published in the DoD/DOE QSM Version 5.2 (CAB Portal Only).
C332a – Specific Checklist: NFPA 790 Field Evaluation Body Accreditation Program is a revised document dated 12/17/18. Editorial change only.
C332b – Specific Checklist: NFPA 791 Field Evaluation Body Accreditation Program is a revised document dated 12/17/18. Non-Editorial for:
• Addition of part 8 to section 5.8.
• Addition of parts 8 and 9 to section 5.11
C346 – Specific Checklist – DOE QSM Version 5.2 Module 1-Proficiency Testing is a revised document dated 1/22/19. Non-Editorial for added new requirements and updated requirements as published in the DoD/DOE QSM Version 5.2 (CAB Portal Only).
Requirements:
R204 – Specific Requirements: Food, Dietary Supplements and Pharmaceutical Testing Laboratory Accreditation Program is a revised document dated 1/8/19. Non-Editorial for: Revision to refer to updated general and specific criteria, name of the document (CAB Portal Only).
R204 – Specific Requirements: Food, Dietary Supplements and Pharmaceutical Testing Laboratory Accreditation Program Transition Memo is a new document dated 1/8/19. Non-Editorial for: Beginning July 1, 2019, all assessments of testing laboratories falling under the A2LA Food Program requirements will be subject to being assessed against the 2018 ALACC Guidelines. Both new applicants and currently accredited laboratories that are due to have assessments prior to July 1, 2019, and that are seeking to be assessed to 2018 ALACC Guidelines before this date may do so by making a request to A2LA at the time of application (CAB Portal Only).
